The Complete Combustion of Which of the Following Substances Produces
While carbon dioxide and water are produced when hydrocarbons burn in a plentiful supply of oxygen complete combustion is not always possible. Carbon Dioxide CO 2 Carbon dioxide is one of the maor products of combustion with fossil fuels since carbon accounts for 6090 percent of the mass of fuels that we burn.
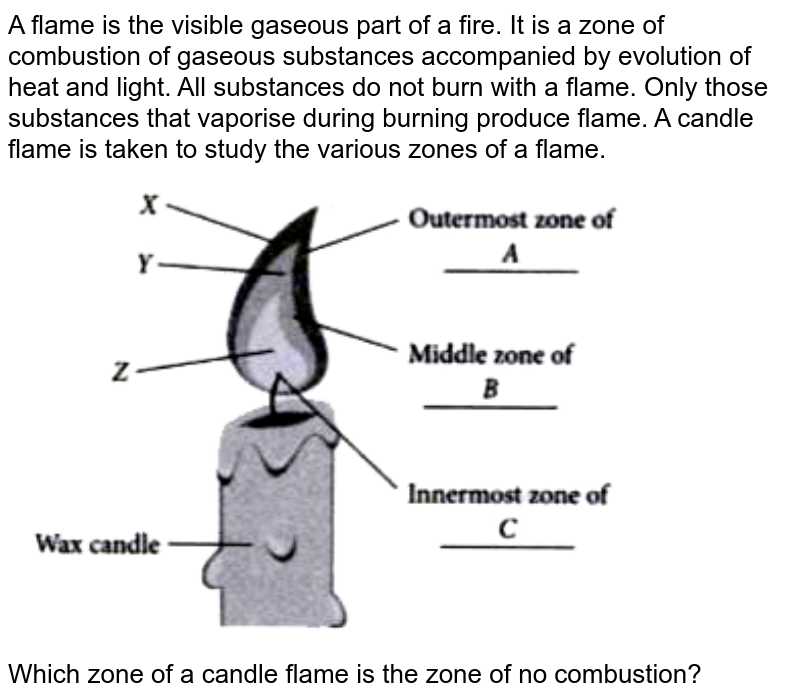
A Complete Combustion B Partial Combustion C Unburnt Vapours
A propane appliance that is functioning properly and producing an ideal burn of hydrocarbon will give off a blue flame.
. Methaneoxygen--carbon dioxidewater Ps2g--P2O5s Which of the following is the correct skeleton equation that takes place when solid phosphorus combines with the oxygen gas to form diphosphorus pentoxide. Ex- iron slowly reacts with oxygen and moisture present in the air to form rust. However a small amount of nitrogen.
New questions in Chemistry. The final substances are called reactants. Carbon fixation involves the addition of carbon dioxide to _____.
If the fuel is. A CaHCO3 B NO C C8H18 D K2CO3. A chemical equation that does not indicate relative amounts of reactants.
The products of a combustion reaction include. Complete Combustion produces carbon dioxide and water as major byproducts. Based on this combustion analysis data what mass of oxygen is from the mass of compound combusted and not from the oxygen gas used to combust the compound.
Bonds between atoms are broken and new bonds are formed. See the answer See the answer done loading. Soda water is a solution of carbon dioxide in water.
Separation of water vapor from the gas stream is simple. Complete combustion is a chemical reaction in which all of the carbon atoms in a particular substance are entirely consumed. Complete Combustion of Hydrocarbons It is the process of burning hydrocarbons in excess of oxygen and yields carbon dioxide and water as a product.
During rusting of iron the heat is released at such a slow rate that it is difficult to detect it but no light is produced. The complete combustion of which of the following substances produces carbon dioxide and water. None of the above.
Balance the equation. The complete combustion of organic compounds produces Carbon Dioxide CO2 Water H2O and a residue of any impurities which were present in the coal before combustion. Complete combustion of hydrocarbons without impurities in the presence of enough oxygen produces water vapor and carbon dioxide.
Combustion reactions must have oxygen as a reactant. A combustion reaction produces this as one of its products. Oxygen should be present in excess and hydrocarbon is used as a limiting reagent to achieve this.
April 26 2022. Complete combustion creates a blue colored flame. Complete combustion has occurred when all the carbon in the product is in the form of carbon dioxide.
Incomplete combustion produces carbon monoxide carbon dust and water as major byproducts. Condensation can provide the suitable separation. Combustion is usually understood to be a synonym for burning though the.
Bonds between atoms are broken and new bonds are formed. The complete combustion of which of the following substances produces carbon dioxide and water. This combustionreaction leads to the formation of 16 molecules of CO2 carbon dioxide and 18 molecules of H2O water.
All chemical reactions can be classified as one of five general types. Think about your result. When the equation for the complete combustion of ethanol C2H5OH is balanced what is the coefficient for oxygen.
Water carbon dioxide and carbon monoxide. The complete combustion of which of the following substances produces carbon dioxide and water. Note that the water produced is in the gas state rather than the liquid state because of the high temperatures that accompany a combustion reaction.
All chemical reactions can be classified as one of five general types. In a double-replacement reaction one of the products. We will now look at six products of combustion.
When elements are burned the products are primarily the most common oxides. In its combustion are envolved 2 molecules of C8H18 octane itself and 25 molecules of O2 oxygen. In precombustion technologies combustion is made using pure oxygen up to 97 purity.
Science Chemistry QA Library The complete combustion of which of the following substances produces carbon dioxide and water. When a hydrocarbon burns in oxygen the reaction will primarily yield carbon dioxide and water. Fuels such as natural gas and petrol contain hydrocarbons.
Which of the following is a balanced equation for the combination reaction that takes place when iron III oxide is formed from. A single reactant is the identifying characteristic of a decomposition reaction. In complete combustion the reactant burns in oxygen and produces a limited number of products.
Combustion of a compound of formula CxHyOz yields 0209 g H2O molar mass 18016 gmol and 0512 g CO2 molar mass 4401 gmol when 0497 g of O2 molar mass 32 gmol is used. No in combustion process light is not produced always. The complete combustion of which of the following substances produces carbon dioxide and water.
This solution is composed of a - Carbon fixation involves the addition of carbon dioxide to _____. This is a compound thats quite popular due to its presence in gasoline. 1164 C 2 H 5 OH l 3 O 2 g 2 CO 2 g 3 H 2 O g Step 3.
Complete combustion needs a plentiful supply of air so that the elements in the fuel react fully with oxygen.

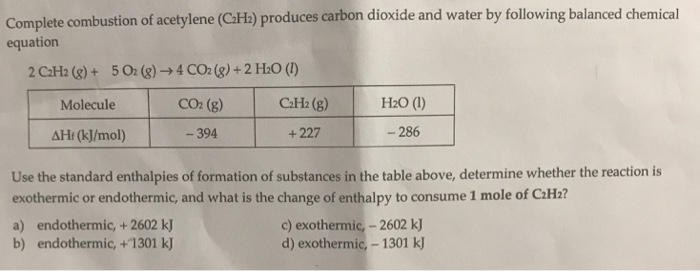
Solved Complete Combustion Of Acetylene C H2 Produces Chegg Com

Inhibitor Easy Science Chemical Reactions Science

Intermediate Easy Science Easy Science Intermediate Chemical Reactions

0 Response to "The Complete Combustion of Which of the Following Substances Produces"
Post a Comment